2a | Neuropsychiatric and rare CNS conditions |
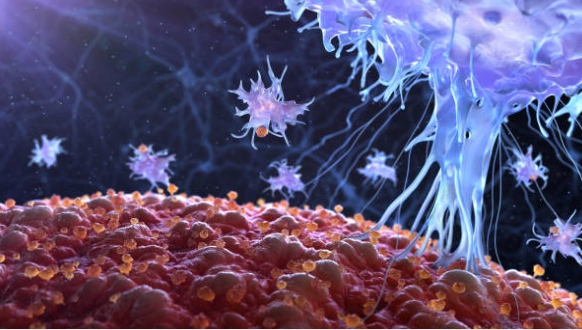
Designed to overcome the poor oral bioavailability of allopregnanolone to advance what we believe could be a best-in-class new medicine for treating anxiety, mood disorders and Fragile X-associated Tremor/ Ataxia Syndrome. Allopregnanolone is a positive allosteric modulator of GABAA receptors that has therapeutic potential across a wide range of neurological conditions, including anxiety and Fragile X-associated Tremor/ Ataxia Syndrome, though its therapeutic application has been limited due to high first pass metabolism. Our Glyph platform reversibly links a drug to a lipid, creating a novel prodrug. We believe this technology has the potential to provide a broadly applicable means of enhancing the bioavailability of certain orally administered drugs that would otherwise be limited by first-pass liver metabolism.
| Phase completed | Phase in progress |
A range of neurological and neuropsychological conditions, including anxiety, mood disorders and Fragile X-associated Tremor/Ataxia Syndrome