See the press release HERE.
Conditions involving inflammation and fibrosis, including idiopathic pulmonary fibrosis
Celea Therapeutics is advancing deupirfenidone (LYT-100) as a potential new standard of care (SOC) for the treatment of idiopathic pulmonary fibrosis (IPF), a progressive and fatal lung disease estimated to affect over 230,000 people across the U.S. and EU52. Deupirfenidone is a next generation antifibrotic and a deuterated form of pirfenidone, one of three FDA-approved therapies for IPF.
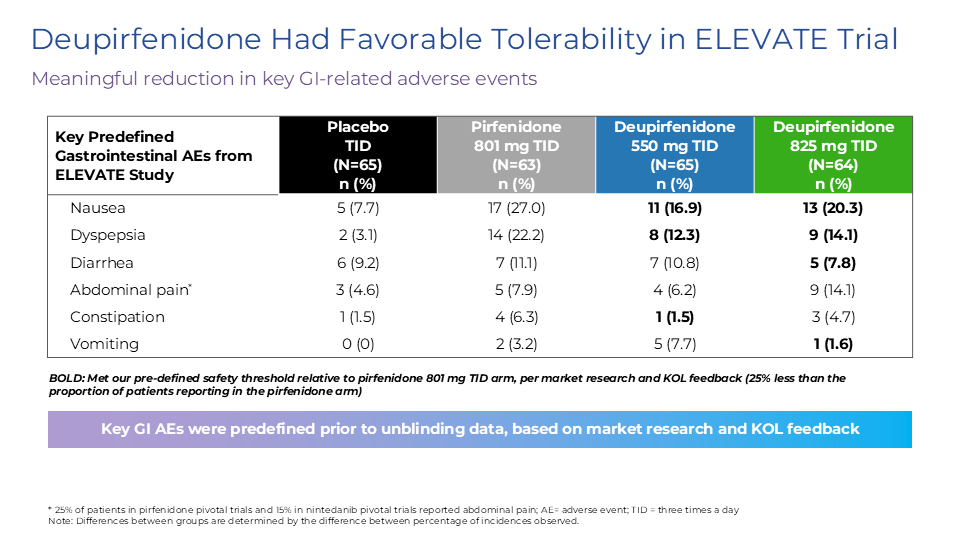
The uptake of and adherence to approved antifibrotics has historically been limited largely due to the tradeoff between tolerability challenges and modest efficacy, and only ~25% of people with IPF in the U.S. had ever received treatment as of 20193.
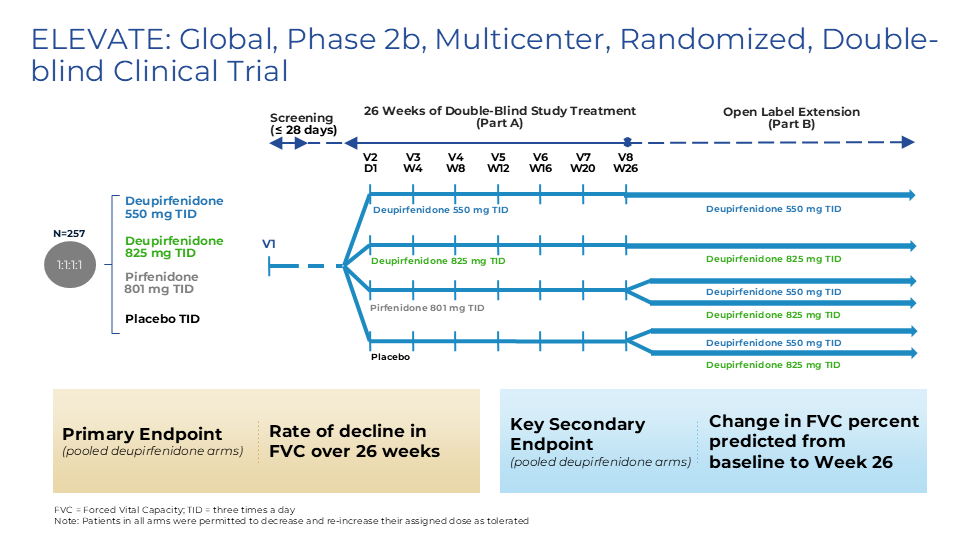
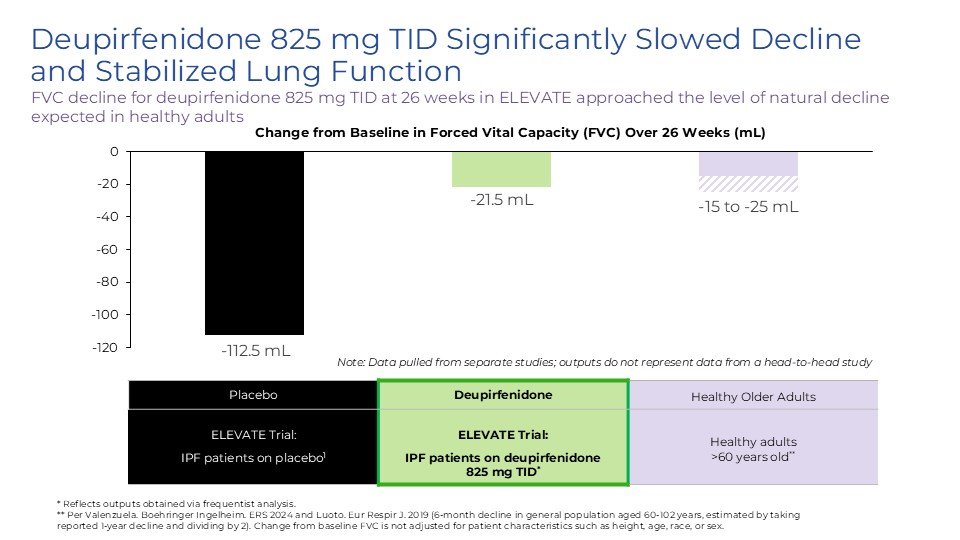
Deupirfenidone may overcome these limitations. In the global Phase 2b ELEVATE IPF trial, deupirfenidone demonstrated the potential to stabilize lung function decline over at least 26 weeks as a monotherapy while maintaining a favorable safety and tolerability profile. Initial data from an ongoing open-label extension study suggest this effect may be sustained through at least 52 weeks. These findings support the potential for deupirfenidone to offer a meaningful advance for people living with this progressive and deadly disease. Beyond IPF, deupirfenidone could also address multiple underserved fibrotic diseases, including progressive pulmonary fibrosis (PPF), also termed progressive fibrotic ILD (PF-ILD).